A Year of Growth and Discovery: Reflections from a Clinical Project Manager at Pharmalys Ltd
Pharmalys Ltd is a regional Contract Research Organisation (CRO) that has seen significant growth over the past three years. Headquartered in Europe with regional offices in Dakar, Pharmalys supports sponsors around the world in conducting safe and high-quality pan-African clinical research. Our mission is to improve health outcomes through data excellence. To achieve this, we operate across several European countries and nearly half of the African continent, always adhering to international standards in health research.
For years, Pharmalys has been committed to advancing clinical research in Africa, driven by the dedication and expertise of our team members. Today, we are excited to share Ndeye’s reflections on her first year with us as a Clinical Project Manager – a year marked by growth, learning, and discovery. Read more: https://www.pharmalys.com/a-year-of-growth-and-discovery-reflections-from-a-clinical-project-manager-at-pharmalys-ltd/
Precision Medicine: An Introduction to Umbrella and Basket Trials
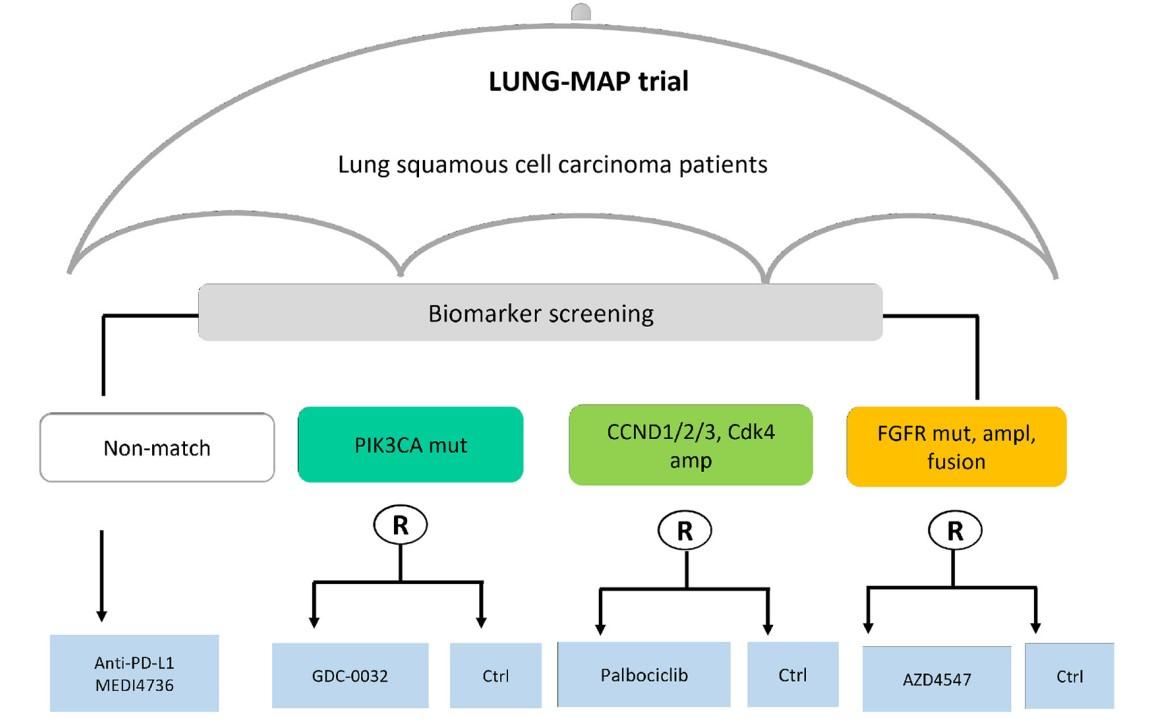
Example of a real umbrella trial. Study scheme of the lung cancer master protocol (Lung-MAP–NCT03851445) trial. From Ouma L.O. et al. (2022) Front. Med. 9:1037439. doi: 10.3389/fmed.2022.1037439
In recent decades, cancer treatment has evolved from relying on non-specific, cytotoxic chemotherapy and radiation to adopting a more targeted approach, driven by advancements in omics technology. As these technologies have become more sophisticated and widely accessible, it’s now possible to tailor treatments to the molecular abnormalities present in each patients’ tumour. Genomic studies have revealed that individual tumours, particularly metastatic ones, exhibit significant complexity and heterogeneity. This means that the most effective therapies must be customised to the individual, fueling the growing interest in precision medicine. Precision medicine aims to enhance treatment outcomes by identifying therapies specifically tailored to the genetic makeup of a patient’s disease. (ie, targeted therapies).
In the United States, momentum toward precision medicine has grown significantly. In May 2018, the National Institutes of Health launched their “All of Us” Initiative, aiming to collect demographic and biological data from over one million people to improve precision care in oncology and other medical fields. Similarly, in September 2018, the US Food and Drug Administration (FDA) released draft guidance for master protocols, signalling their support for wider adoption of precision-based research methods. In the United Kingdom, the National Health Service’s 2019 Long-Term Plan highlights precision oncology as a key area of focus.
The rising interest in precision care underscores the importance of biomarkers in the development of targeted therapies. One of the most notable methodological advancements in biomarker-guided clinical trials has been the development of master protocols, which are overarching trial designs that evaluate multiple hypotheses. These protocols aim to improve efficiency by standardising trial procedures. Two primary types of master protocols are basket and umbrella trials. Read more: https://www.pharmalys.com/precision-medicine-an-introduction-to-umbrella-and-basket-trials/

Call for Public Review: Updated Guidance on Inclusive Language in Medical and Scientific Publications
In the realm of medical and scientific publishing, the precision and respect embedded in our language are crucial. The choice of terminology when describing individuals and groups can profoundly influence the clarity and fairness of our communications. Inclusive language is not merely a stylistic preference but a fundamental aspect of respectful and accurate representation.
To advance these principles, the AMA Manual of Style committee has revised its guidance on the use of inclusive language, focusing on gender, sex, gender identity, sexual orientation, and age. This updated draft is designed to help authors and editors navigate the complex landscape of demographic reporting with sensitivity and consistency. Read more and access the guidance: https://www.pharmalys.com/call-for-public-review-updated-guidance-on-inclusive-language-in-medical-and-scientific-publications/











