Beginning of October 2023, Dr. Karim Bagaté, Director of Clinical Operations & Safety, and Mr. Assane Ndiaye, Clinical Research Associates Manager at Pharmalys delivered a capacity building programme, named ERC (Ethics and Regulatory Capacity) in The Gambia. The objective of this advanced workshop was to equip senior members of the National Ethics Committee for Life Sciences and Health authorities with the knowledge and skills they need to conduct high-quality ethics reviews, ensure optimal protection of research participants, and effectively manage and coordinate clinical trials. Karim and Assane provided a one-week intensive training course on Good Clinical Practice (GCP) and performed a clinical trial inspection. The training covered many topics from the drug development process and history of ICH GCP, to fraud, misconduct & research integrity, including the management of the investigational product, drug safety, and quality assurance to name but a few.
During the 2-day clinical trial site mock inspection, Pharmalys team ensured the staff were working in accordance with the protocol and GCP guidelines. They made a document inspection consisting of a document review to check the availability, completeness, authenticity, and GCP compliance of the documents, interviews with the investigational team to assess the site organisation and the team training, and a site visit to evaluate the organisation of the various sites.
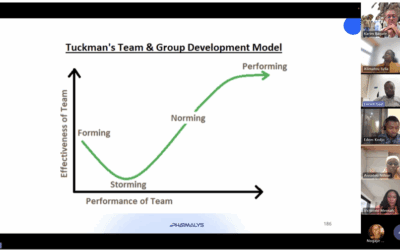
Project Management Training: Pharmalys’ Commitment to Professional Growth
Pharmalys continues to invest in the professional development of its employees through dedicated training initiatives. A recent initiative is the Project Management Training Programme (PMBOK concept), led by Dr. Karim Bagaté, Director of Clinical Operations and Safety...